6. GENERAL PRINCIPLES & PROCESSES OF ISOLATION OF ELEMENTS – LONG ANSWER TYPE QUESTIONS
6. GENERAL PRINCIPLES & PROCESSES OF ISOLATION OF ELEMENTS
Q. 1. Describe the prepare of Ammonia from Haber’s process ? And also give the condition to produce maximum amount of Ammonia according to Lechtlier’s principle ?
Ans⇒ On a large scale Ammonia is produced by chemical combination of Nitrogen and hydrogen, according to following reaction :
N2 (g) + 3H2 (g) ![]() 2NH3 (g)
2NH3 (g)
ΔHr = – 46.1 KJ mole-1
Lechatlier’s principle : In a reversible reaction once equilibrium is established after that any of factor is brought to change then equilibrium shifts in that direction so as to destroy the change. There are following condition under which we get maximum amount of ammonia :
(i) Effect of concentration : If we increase the concentration of Nitrogen and hydrogen gas or take out some amount of concentration of ammonia at equilibrium then it favours forward reaction and production of ammonia will increase.
(ii) Effect of temperature : Since the reaction is exothermic so the high production of ammonia will favour at optimum low temperature hence the optimum temperature is 720 k.
(iii) Effect of pressure : Since the production of ammonia takes with the decrease in number of moles, so on increasing pressure it favours forward direction and more amount of Ammonia is pressure. The optimum pressure required for this reaction is 200 atmospheric pressure.
(iv) Effect of catalyst: Catalyst used is Iron filling (Fe2O3) and Molybdenum (Mo) as permoter. A catalyst enhances the rate of reaction. It does not the value of equilibrium constant (kc).
Q. 2. How iron is extracted from Hematite ore is blast furnace gives the reaction involved at different stages of blast furnace and also discuss the metallurgy involved ?
Ans⇒ Hematite ore (Fe2 O3) is ore of Iron which have mainly Silica (SiO2) as main impurity. Hematite ore (Fe2O3) is put in Blast furnace along with coke (c) and limestone.
There are different steps or stages for metallurgy first ore is calcined, roasted and then reduced or smelled to get pure iron from blast furnace (It is a double cup and cone arrangement).
Calcination : The ore is calcined first to remove earthly impurity and change fereous oxide into ferric oxide.
Roasting : The ore is roasted to remove voltaile impurity, to make the ore poreous and roasted ore is easily reduced.
4FeO + O2 (g) → 2Fe2O3
S+O2 (g) → SO2 (g) ↑
4AS + 3O2 (g) → 2As2O3(g) ↑
The following reaction takes place at blast furnace iron ore is put along with coke, limestone. In Blast furnace the reaction takes at lower zone (zone of fusion), middle zone and upper zone.
The reaction taking place at blast furnace is
C (s) + O2 → CO2 (g)
This is the reaction taking place at lower zone due to burning of coal with air and produces CO2 (g). The temperature at this zone is 2170 k.
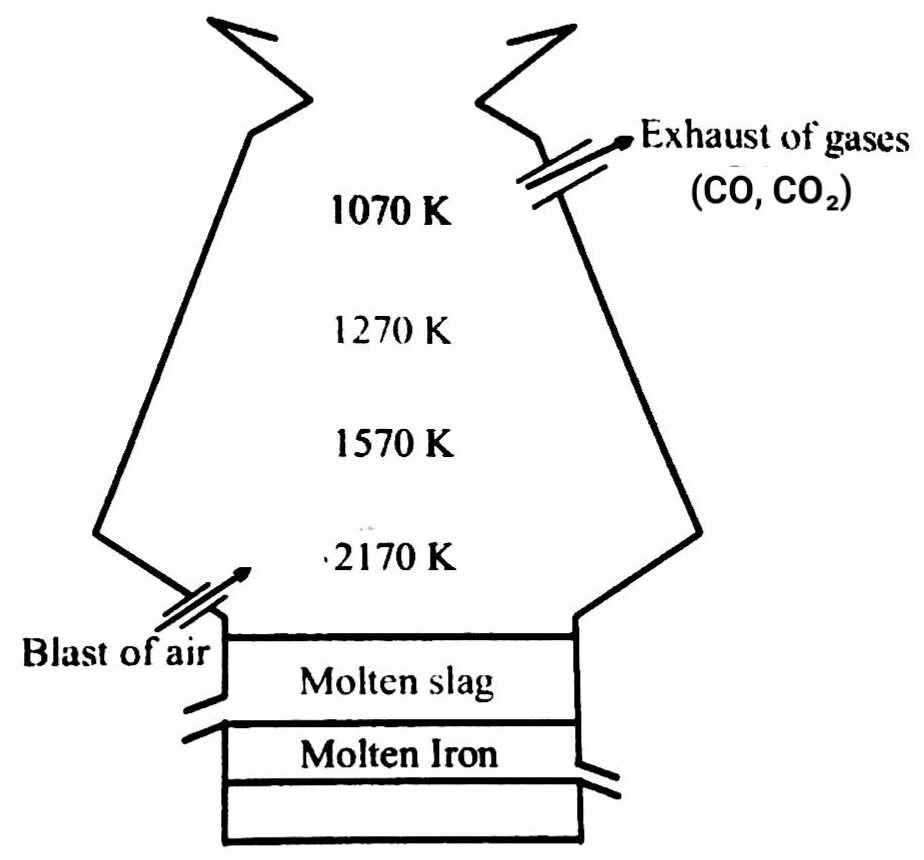
The reaction taking place at middle zone is
CaCO3 (s) → CaO (s) + CO2 (g)
Lime stone
CaO + SiO2 → CasiO3 (s)
Flux + Gangue Slag Calcium Silicate
The coke chemically combines with CO2 (g) to gives Carbon monoxide and this reaction is endothermic reaction which lower down the temperature of blast furnace at this zone.
C (s) + CO2 (g) → 2CO (g)
The reaction at upper zone in the temperature range 800-1100 k.
3Fe2O3 (s) + CO (g) → 2Fe3O4 + CO2 (g)
Fe3O4 + CO (g) → 3FeO + CO2 (g)
FeO (s) + CO (g) → Fe (s) + CO2 (g)
Carbon Mono oxide produced in the middle zone acts as reducing agent and reduced hematile in iron.
In the blast furnace the molten slag of Casio, (Calcium silicate) floats on Iron through different opening we separate it.
So the Iron obtained from blast furnace contains about 4% carbon is known as pig iron and cast into variety of shape.
Q. 3. Explain : (i) Zone refining, (ii) Column Chromatography.
Ans⇒ (i) Zone refining : The method is based on the principle that the impurities are more soluble in the melt than in the solid state of the metal. A circular mobile heater is foixed at one end of a rod of impure metal. A molten zone moves along with the hater which is moved forward. As the heater moves forward, the pure metal crystallizes out of the metal and the impurities pass on into the adjacent molten zone. The process is repeated several times and the heater is moved in the same direction. At one end impurities get concentrated. This end is cut off. In this way, the impurities are swept from one end of the bar to the other. By repeating the process ultra pure metal of silicon, germanium etc are obtined.
(ii) Column Chromatography : In column chromatography an adsorbent (e.g. Al2O3) is packed in
a glass column. The mixture to be separated or purified, taken a suitable solvent, is applied on the top of the column. The components of the mixture get adsorbed on the column. They are then eluted out with the suitable eluent (Solvent). The weakly adsorbed component is eluted first followed by the more strongly adsorbed and so on. The method is especially suitable for such elements which are available only in minute quantities and the impurities are not very much different in their chemical behaviour from the element to be purified.
Q. 4. Outline the principles of refining of metals by the following methods :
(i) Electrolytic refining (ii) Vapour phase refining
Ans⇒ (i) Electrolytic refining : The impure metal is made the anode and a pure strip of the same metal the cathode in a suitable electrolyte both.
Anode : M → Mn+ + ne–
Cathode : Mn + ne– → M
The net result is the transfer of pure metal from the anode to the cathode. The applied voltage is such that more electropositive metals (impurity) remain as ions in the bath whereas the less electropositive metals (impurities) remain unionized and fall done as anode mud.
(ii) Vapour phase refining : (a) Mond process : Nickel when heated in a stream of carbon monoxide forms volatile nickel carbonyl, Ni(CO4). The carbonyl vapour when subjected to still higher temperature undergoes thermal decomposition giving pure metal.
Ni + 4CO ![]() Ni(CO) 4
Ni(CO) 4 ![]() Ni + 4CO Impure pure
Ni + 4CO Impure pure
(b) Van Arkel Process : Zirconium (or titanium) is heated in iodine vapour at about 870 K to form volatile ZnI4. The latter when heated over a tungesten filament at 2975K decomposes to give pur zirconium.
Zr + ZI2 ![]() ZnI4
ZnI4
(Impure) I2 (Vaspir) (vapour)
![]() Zr + 2I2
Zr + 2I2
. Pure
Q. 5. Name three ores which are concentrated by froth-floatation process. What is meant by depresent ?
Ans⇒ The three ores which are concentrated by froth floatation process are : (i) Copper pyrites (CuFeS2). (ii) Zinc Sulphide (ZnS). (iii) Silver glance (Ag2 S).
Depressants are used to prevent certain types of particles from forming the froth with bubbles.
Example : NaCN is used as a depressant in the separation of ZnS and PbS ore. NaCN forms a layer of Zinc complex Na2Zn (CN)4 on the surface of ZnS and thereby prevents it from formation of froth.
Q. 6. Write the conditions to maximise the yield of H2SO4 by contract process.
Ans⇒ Conditions are : (i) High concentration of reactants.
(ii) Low temperature: But an optimum temperature of 623-723 K must be maintained.
(iii) High pressure : Normally a pressure of about two atmosphere is maintained.
(iv) Presence of catalyst : In order to accelerate the reaction, the presence of catalyst is quite helpful. V2O5 is used as a catalyst.
(v) Purity of gases : Gases must be completely free from dust and poisonous gases like arsenic oxide before they are passed through the catalyst.
Q. 7. Write down the reactions taking place in different zones in the blast furnace during the extraction of iron.
Ans⇒ Near the layers, coke and lime stone are heated when C burns to give CO2.
C(s) + O2 (g) → CO2 (g); ΔH = – 393.3 kj mol-1
Since the reaction is exothermic, lot of heat is produced and the temperature here is 1673 K.
As the gases move up, they meet the descending charge. The coke present in the charge reduces CO2 to CO.
CO2+ C → 2CO ↑ ; ΔH + = + 163.2 kJ
Since the reaction is endothermic, therefore, the temperature falls gradually to about 1423 K.
Below 1123 K, CO reduces the ores to FeO.
Fe2O3+ CO ![]() 2FeO + CO2 ↑
2FeO + CO2 ↑
Reduction of Feo to Fe by CO occurs at about 1123 K.
FeO + CO ![]() Fe + CO2 ↑
Fe + CO2 ↑
In this region lime stone also decomposes to form CaO and CO2.
CaCO3 (s) → CaO (s) + CO2 (g)
CaO (s) + SiO2 → CaSiO3
Silica (fusible slag)

