
13. AMINES – LONG ANSWER TYPE QUESTIONS
13. AMINES
Q. 1. Explain the following:
(i) Aniline dissolves in aqueous HCl.
(ii) Amines are stronger bases than ammonia.
(iii) Aniline is a weak base than ethylamine.
(iv) The amino group in ethylamine is basic whereas that in acetamide is not basic.
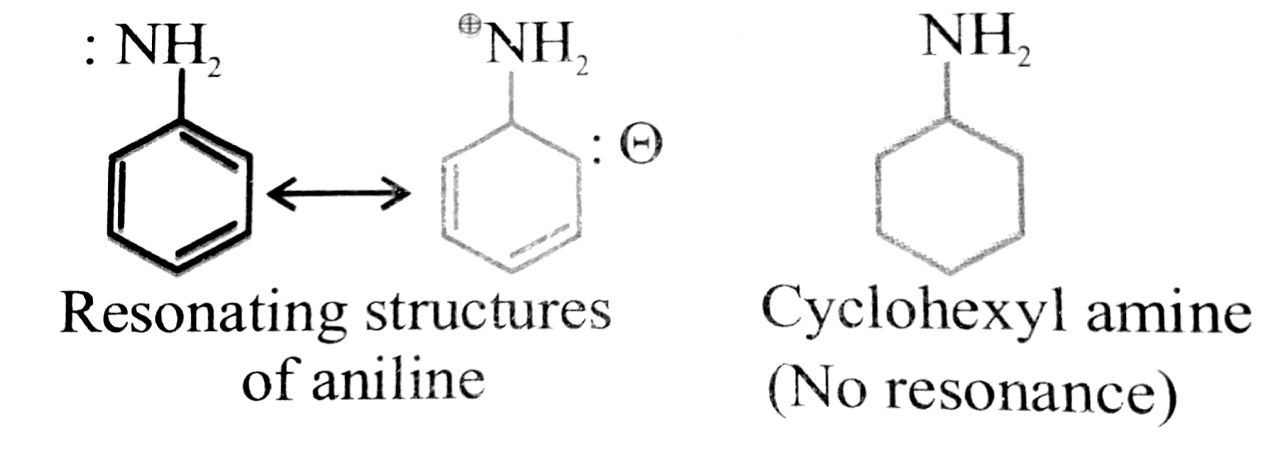
(v) Cyclo hexylamine is a stronger base than aniline.
Ans⇒ (i) The solubility of C6H5NH2 in dil. HCl is due to the formation of water soluble salt.
C6H5NH2 + H3+ + Cl– → C6H5NH3+Cl– + H2O Anilinium chloride
(ii) Amines have electron repelling group which increase the electron density on nitrogen, while ammonia has no such group.
(iii) In aniline the electron pair of nitrogen atom is delocalised due to resonance and hence lesser available for protonation while ethylamine does not undergo resonance.
(iv) In amides the lone pair of electrons on nitrogen atom is delocalised and hence less available for protonation than in amines where no resonance is possible and thus
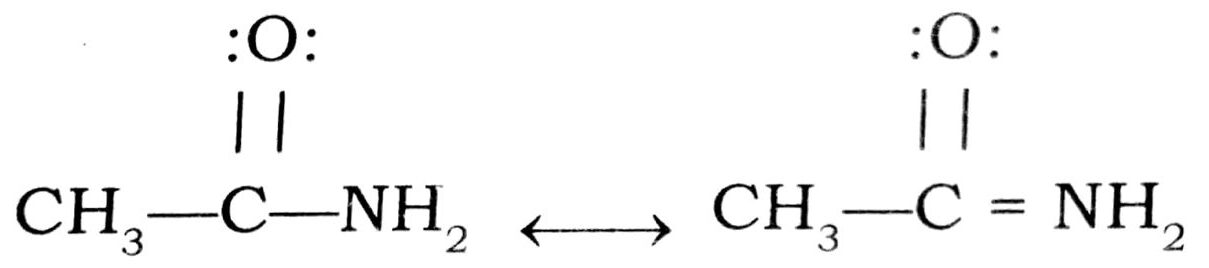
acetamide is a weaker base than ethylamine.
(v) Aniline is weaker base than cyclohexylamine because of resonance while there is no resonance in cyclohexylamine.
Q. 2. (i) Write str amines corresponding to t C4H11N.
(ii) Write IUPAC names of
(iii) What type of isomeri different pairs of amines?
Ans⇒ Eight isomeric amines are Possible

Q. 3. Arrange the following in increasing order of their basic strength:
(i) C2H5NH2, C6H5NH2, NH3, C6H5CH2NH2 and (C2H5)2NH
(ii) C2H5NH2, (C2H5)2NH, (C2H5)3N, C6H5-NH2
(iii) CH3NH2, (CH3)2 NH, (CH3)3N, C6H5NH2, C6H5 CH2NH2
Ans⇒ (i) (C2H5)2NH > C2H5NH2 > C6H5CH2NH2 > NH3 > C6H5NH2
(ii) (C2H3)2NH > (C2H5)3N > C2H5NH2 > C6H5NH2
(iii) (CH3)2NH > CH3NH2 > (CH3)3N > C6H5CH2NH2 > C6H5NH2
Q. 4. Write common name of the following compounds :
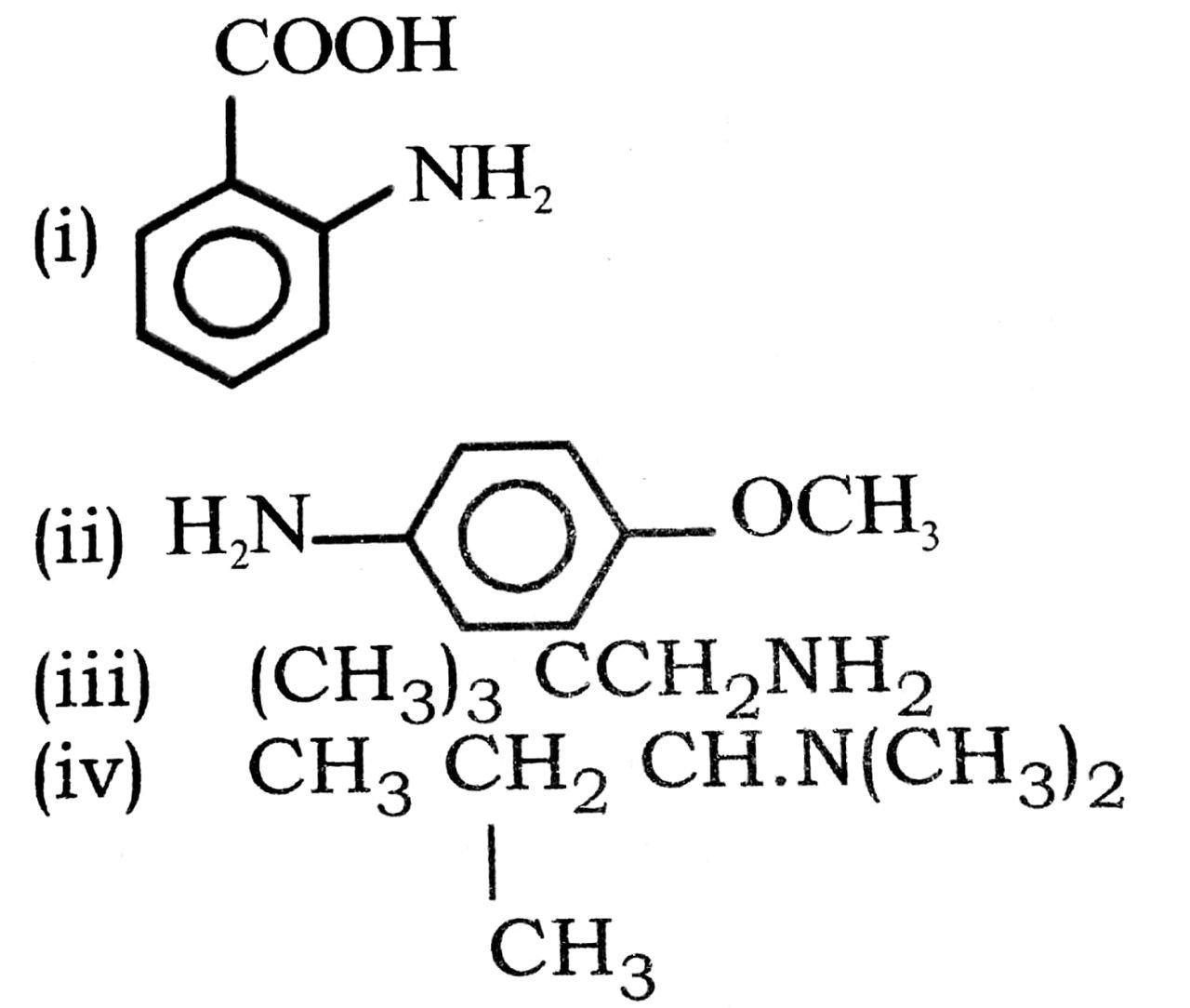
Ans⇒ (i) o-amine benzoic acid.
(ii) p-methoxy aniline (or p-anisidine).
(iii) neopentylamine.
(iv) Sec-butyl dimethy lamine.
Q. 5. Explain the following reactions by taking one suitable example in each case :
(i) Hoffman’s bromamide reaction
(ii) Gattermann’s reaction
(iii) Carbylamine reaction
(iv) Diazotisation
(v) Coupling reaction
(vi) Ammonolysis
(vii) Acetylation
(viii) Gabriel phthalimide synthesis
Ans⇒ (i) Hoffman’s Bromamide Reaction : When an amide (aliphatic or aromatic) is treated with bromine in alkali solution, it is converted to a paimary amine that has one carbon atom less than the starting amide.
RCONH2 ![]() RNH2 + Na2CO3 + 2NaBr + 2H2O Aliphatic Amide
RNH2 + Na2CO3 + 2NaBr + 2H2O Aliphatic Amide
. Amine
C6H5CONH2 ![]() C6H5NH2 + Na2CO3 + 2NaBr + 2H2O
C6H5NH2 + Na2CO3 + 2NaBr + 2H2O
Aromatic amide Aniline
(Benzamide)
CH3CONH2 ![]() CH3NH2
CH3NH2
Ethanamide Methanamine
This reaction is extremely useful for stepping down (or descending) homologous series.
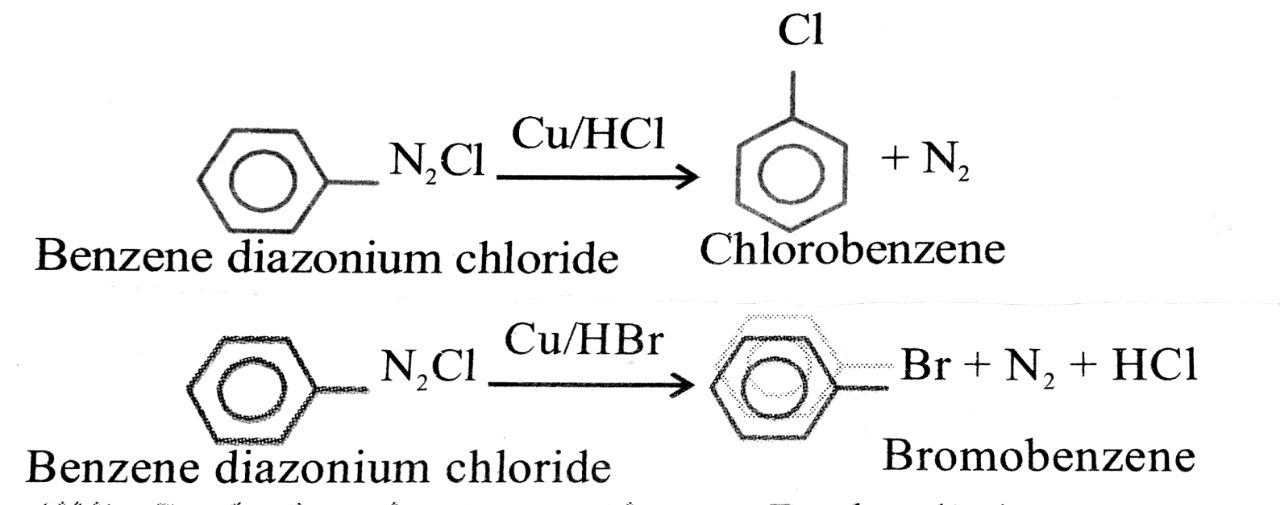
(ii) Gattermann’s reaction: When benzene ring and diazonium salt is treated with Cu/HCl or Cu/HBr, chlorobenzene or bromobenzene is formed. The yield is about 40%.
(iii) carbylamine reaction : Both aliphatic and aromatic primary amines when warmed with chloroform and an alcoholic solution of KOH produce isocyanides or carylamines which have an unpleasant odour.
R-NH2 + CHCl3 + 3 KOH (alc.) ![]() R-N
R-N ![]() C
C
.1° amine +3KCl + 3 H2O
In contrast, secondary and tertiery amines (both aliphatic and aromatic) do not give this test.
(iv) Diazotisation : The reaction of converting aromatic primary amines into diazonium salts with a cold (273-278K) solution of nitrous acid is called diazotisation.
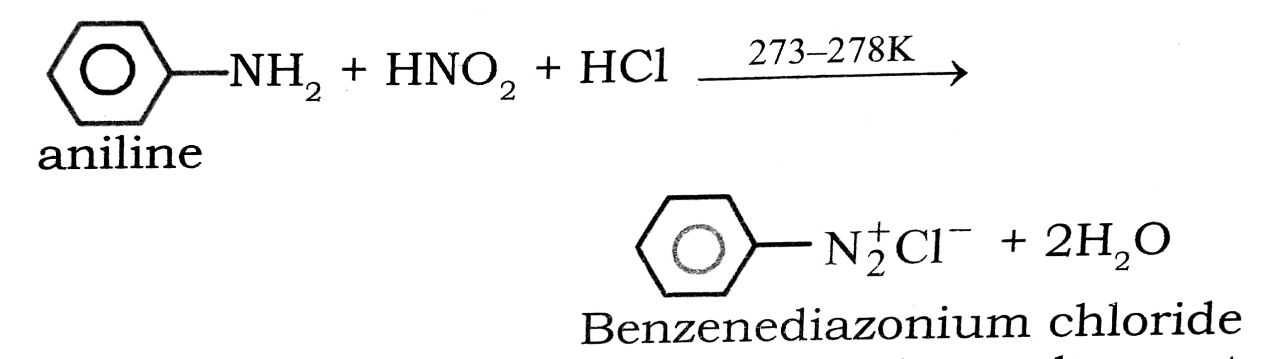
(v) Coupling Reaction : Arenediazonium salts react with highly reactive (i.e., electron-rich) aromatic compounds such as phenols and amines to form brightly coloured azo compounds.
Ar – N = N – Ar.

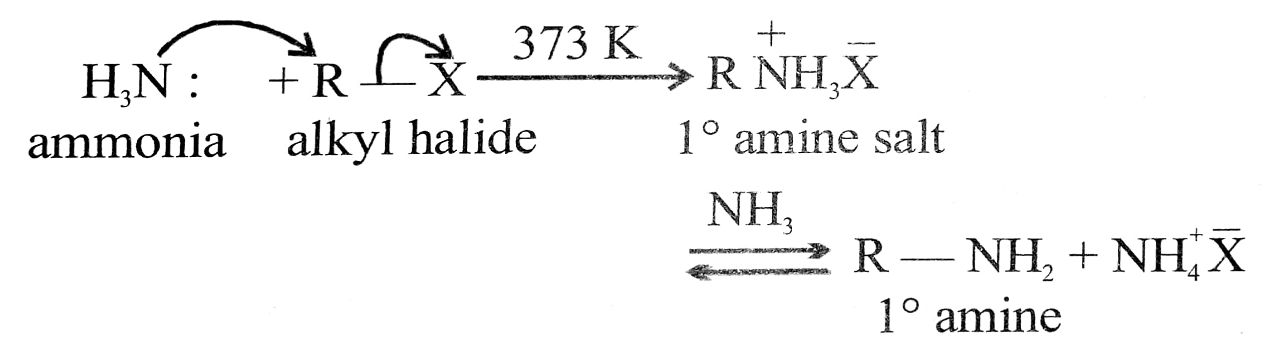
(vi) Ammonolysis : Reactions of an alkyl halide (R – X) with an alcoholic solution of ammonia giving a mixture of 1°, 2°, 3° amines and quaternary salt is called ammonolysis.
R – X + HNH2 ![]() R – NH2 + HX
R – NH2 + HX
(excess) (Chiefproduct)
If R – X is used in excess, a mixture of 1°, 2°, 3° amines along with some quarternary ammonium salts is obtained.
![]()
It is a typical nucleophilic substitution reaction.
(vii) Acetylation : The replacement of an active hydrogen of alcohols, phenols or amines with an acyl group (RCO) to form the corresponding esters or amides is called acetylation. Acetylation is carried out in the presence of a base like pyridine, dimethylaniline etc. by acetyl chloride or acetic anhydride.
CH3COCl + C2H5OH ![]() CH3COOC2H5+ HCl
CH3COOC2H5+ HCl
Acetyl chloride ethanol Ethyl acetate
.(CH3CO)2O + C2H5NH2 → CH3CONHCH2CH3 + CH3COOH
Acetic Ethylamine N-Éthylacetamide anhydride
(viii) Gabriel’s phthalmide synthesis : In this reaction phthalimide is converted into its potassium salt by treating it with alcoholic KOH. Then potassium phthalimide is heated with an alkyl halide to yield on N alkyl phthalimide which is hydrolysed to phthalic acid and a primary amine by heating with HCl or KOH solution. This synthesis is very useful for the preparation of pure aliphatic primary amines.
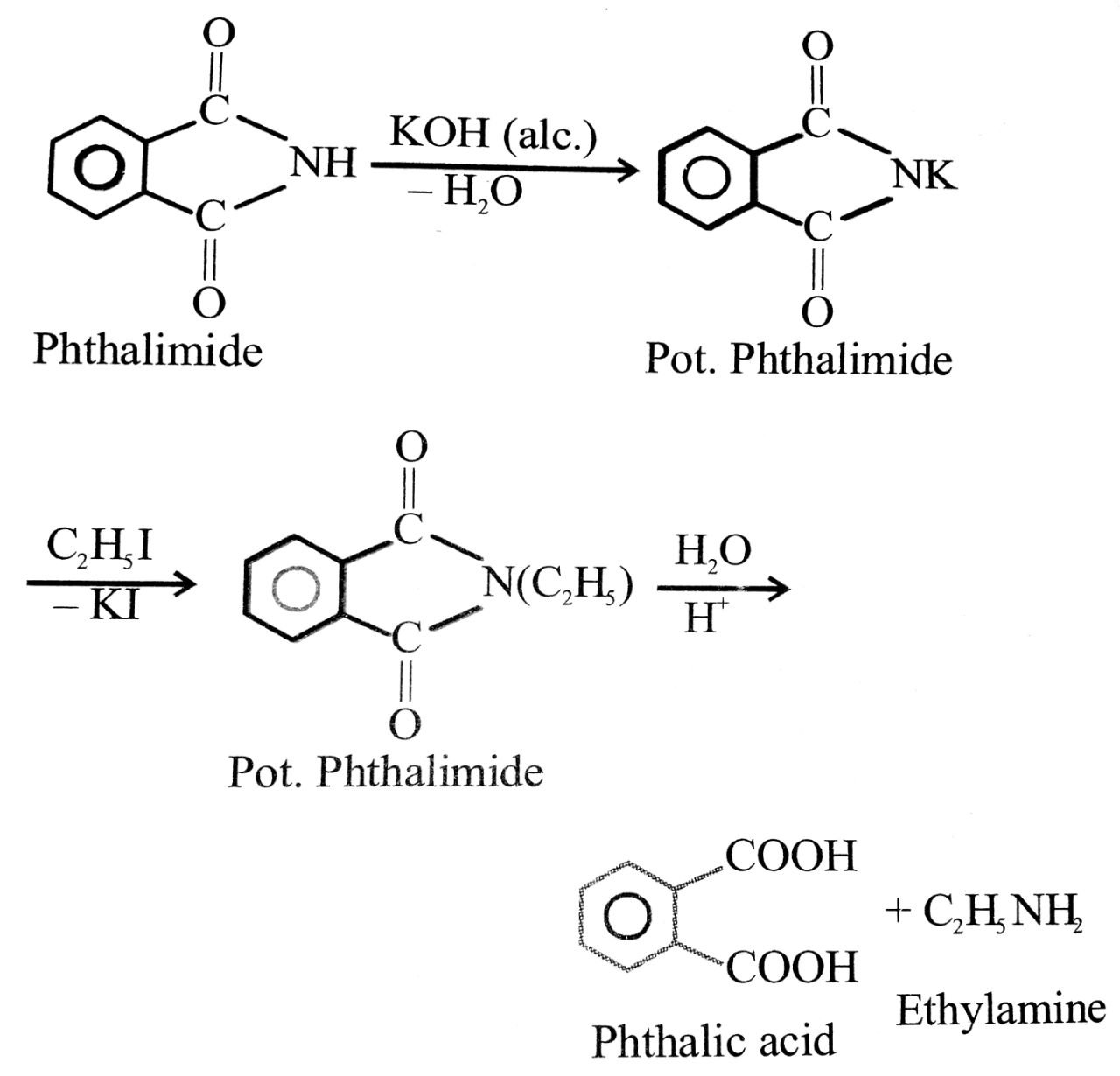
Q. 6. How is anilline prepared commercially ? Describe carbylamine test for primary amines.
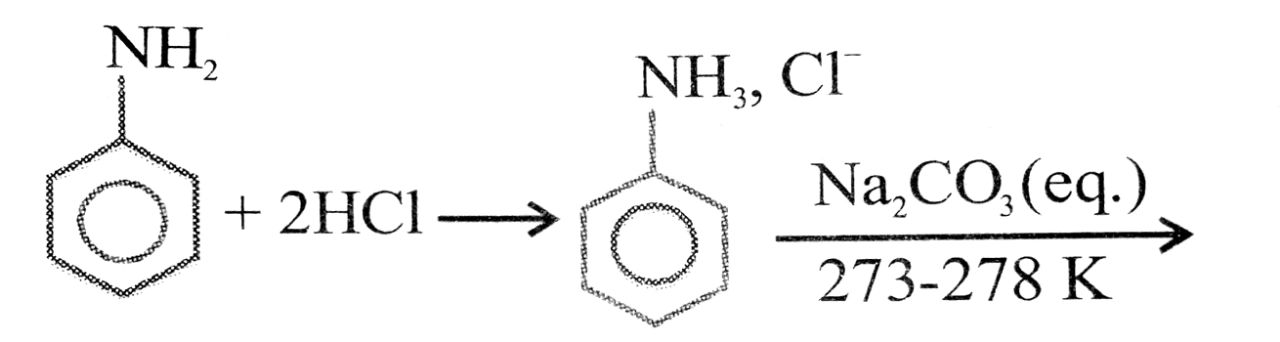
Ans⇒ Aniline is prepared commercially by the reduction of nitrobenzene using iron and hydrochloric
acid.
Fe + 2HCl → FeCl2 + 2H] × 3
C6H5NO2 + 6H → C6H5NH2 + 2H2O![]()
C6H5NO2 + 3Fe + 6HCl + C6H5NH2 + 3FeCl2 + 2H2O
(Nitrobenzene) (Aniline)
Because of the presence of hydrochloric acid in the reaction mixture actually aniline hydrochloride (C6H5NH3 + Cl–) is obtained. This on treatment with aqueous soudium carbonate gives aniline.
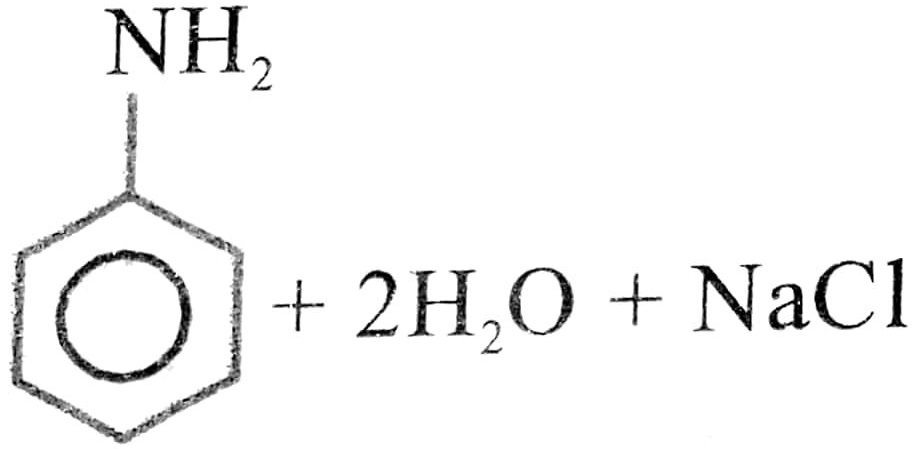
Q. 7. Write IUPAC name of following and classify them into primary, secondary and tertiary amines :
(i) (CH3)2 CHNH2
(ii) CH3(CH2)2NH2
(iii) CH3NHCH(CH3)2
(iv) (CH3)3 CNH2
(v) C6H5NHCH3
(vi) (CH3CH2)2NCH3
(vii) m-BrC5H4NH2.
Ans⇒ (i) (CH3)2 CHNH2 : 1-amino-1-methyl propane (primary)
(ii) CH3 (CH2)2 NH2
1-amino propane (primary)
(iii) CH3NHCH(CH3)2
methyl isopropyl amine (secondary)
(iv) (CH3)3 CNH2
tert-butylamine (primary)
(v) C6H5NHCH3
N-Methyl benzenamine (secondary)
(vi) (CH3CH2)2 NCH3
Diethyl methyl amine (tertiary)
(vii) m-Br CGH NH2
3-isobromo aniline (primary)
Q. 8. How will you prepare :
(i) Benzylamine by Gabriel Pthalimide synthesis.
(ii) Benzylamine from benzene ?
Ans⇒

